The research group of Beijing Institute of Technology has made important progress in the research of anti-inflammatory and analgesic natural products
Release date: 2024-10-18 Contributed by: School of Chemistry and Chemical Engineering Photography: School of Chemistry and Chemical Engineering
Editor: Tian Liu Review: Zhenhua Wang Number of readings:
On October 9, the research group of Professor Liang Jianhua, Key Laboratory of Pharmaceutical Molecular Science and Preparation Engineering, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, made important progress in the research of anti-inflammatory and analgesic natural product drugs。相关成果以“Discovery of glycosidated glycyrrhetinic acid derivatives: natural product-based soluble epoxide hydrolase inhibitors”为题发表于药物化学顶级期刊《冰球突破》。In this work, we designed and synthesized a series of derivatives with triterpenoid natural products 18β-glycyrrhetinic acid and 18α-glycyrrhetinic acid as the backbone, which showed anti-inflammatory and analgesic activities as soluble epoxide hydrolase (sEH) inhibitors in mice。In order to improve the activity and drug-like properties in vivo, the glycoylation of different monosaccharides was further studied. Among them, 3-O-mannose derivative 49Cα (LQ-38) can effectively relieve carrageenan induced foot swelling, acetate-induced pain and writhing and acute pancreatitis in mice。
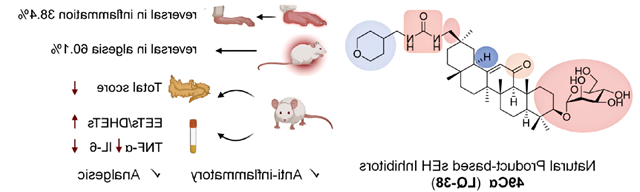
图1. Study on structure-activity relationship of triterpene skeleton
Clinically available NSaids are the main drugs used to treat inflammation and pain, but long-term use may lead to bleeding from gastric and duodenal mucosa ulcers, cardiovascular disease, etc。Because of the imperfection of the existing NSaids, it is an urgent problem to find a new mechanism of anti-inflammatory analgesics。Epoxide eicosatrienoic acid (EETs) is a bioactive metabolite of arachidonic acid produced by CYP (cytochrome P450) enzyme, which has been shown to have anti-hypertensive, anti-inflammatory and other cardioprotective effects。Endogenous EETs are rapidly metabolized to inactive or less active dihydroxy-eicosatrienoic acids (DHETs) by sEH (soluble epoxide hydrolase), and the use of sEH inhibitors is an effective way to stabilize endogenous EET levels and enhance their beneficial effects。In healthy mammals, there is homeostasis between EETs and DHETs。When triggered by external factors such as viral invasion or physical injury, this balance is disrupted, leading to inflammation and pain。
Currently, three sEH inhibitors for different indications have entered clinical studies, but only EC5026 is still in clinical studies (phase I).。However, there is still no significant progress in the research and development of sEH inhibitors based on natural products。Natural products are an important source of clinically effective drugs with a wide range of pharmacological effects and the potential to circumvent drug tolerance caused by target inhibition。Based on the structure-activity relationship of triterpenoids, we conducted iterative design optimization, and finally found that C-30 ureyl glycyrrhetinic acid derivatives had excellent sEH inhibitory activity in vitro, while C-20 or C-3 ureyl derivatives had no inhibitory activity.However, the deficiency is that its body activity is low。Therefore, in order to improve the bioavailability in vivo, we further investigated the effects of different monosaccharide and glycoside bond configurations on 3-OH and the stereoconfiguration at C-18 position on the activity in vivo。The 18α-glycyrrhetinic acid mannose derivative 49Cα containing αglucoside bond was found,It showed good efficacy in relieving foot swelling induced by carrageenan and pain twisting induced by acetic acid.meanwhile,Compound 49Cα regulates the ratio of EETs to DHETs,It shows potential for the treatment of acute pancreatitis。To study the mode of action,We cultured the co-crystallization of sEH with the candidate compound 49Cα,The molecular structure of the complex shows that,The key pharmacophore urea group is bonded to amino acid residues in the sEH tunnel in the form of hydrogen bonds,Role of triterpenoid skeleton and hydrophobic sEH channel,The mannose component extends beyond the sEH channel。

图2. Cocrystalline molecular structure of 49Cα and sEH
49Cα, a glucosidated derivative of mannose utilizing 18α-glycyrrhetirrheinic acid, has been demonstrated to have significant in vivo efficacy in acute pancreatitis models. In addition, 49Cα shows good oral absorption and enrichment in the liver (the main expression tissue of sEH)。Pharmacokinetic studies have shown that it will be hydrolyzed in the body to obtain glycosylated compounds, which is a prodrug;This may be related to the fact that glycosylation increases the solubility of drug molecules in water, which in turn increases the activity in vivo by enhancing absorption。
This research was supported by the Science and Technology Innovation 2030-Brain Science and Brain-Like Research Major Project and the National Natural Science Foundation of China。
About the author:
First authors: Qian Liu and Yixin Wang, Master graduates of the School of Chemistry and Chemical Engineering, Beijing Institute of Technology; Zihao Ge, Beijing University of Chemical Technology; and Minzhen Zhu, postdoctoral fellow of Pazhou Laboratory, Guangzhou。
Corresponding author: Liang Jianhua, Professor, Beijing Institute of Technology, Deputy Director of the Key Laboratory of Pharmaceutical Molecular Science and Pharmaceutical Engineering of the Ministry of Industry and Information Technology. His research interest is the development of new drugs related to major diseases.Zhu Xinhong, National Jieqing, Director of Pazhou Laboratory Brain Disease and Health Research Center, research interest in the pathogenesis and intervention of mental diseases;Feng Yue is a professor at Beijing University of Chemical Technology, whose research interests include the mechanisms of drug action。Professor Li Chun from Tsinghua University also provided design guidance for the project。
Share to:
